BACKGROUND
Management of the hematologic malignancy multiple myeloma (MM) requires a sequence of treatment decisions across the course of the disease to extend survival and maximize quality of life (QoL). With the introduction of novel and increasingly effective therapies and the consequent evolution of treatment paradigms, these decisions have become more complex and personalized. This study aimed to explore how patients living with MM are navigating a rapidly changing care journey.
METHODS
A non-interventional, qualitative study was conducted in adult patients with MM in the United States. Individuals were recruited through the Gryt Health Cancer Community and social media. Data collection centered on a virtual semi-structured interview. Participants were assigned to a group based on refractoriness to at least one proteasome inhibitor, immunomodulatory drug, and/or anti-CD38 monoclonal antibody (non-refractory; single or double refractory; triple refractory). Qualitative data were coded and analyzed for salient themes. This study was reviewed and approved by an Institutional Review Board.
RESULTS
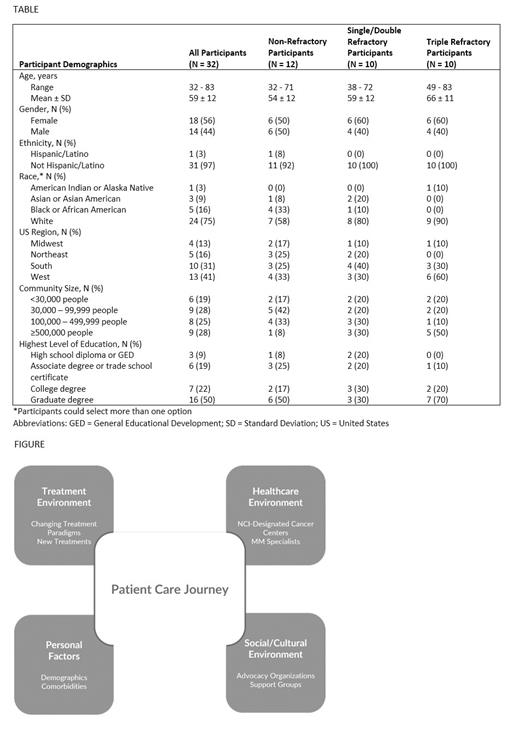
Thirty-two participants (12 non-refractory; 10 single or double refractory; 10 triple refractory) completed the study (Table). Participants were diagnosed between 1998 and 2021 (median: 2017); 38% had been diagnosed within the past 5 years. Data related to the care journey clustered around four key environments: treatment, healthcare, social/cultural, and personal factors (Figure).
Important shifts were observed in the treatment environment. Notably, 34% (N = 11) of participants were currently off treatment (5 non-refractory; 1 double refractory; 5 triple refractory). Participants with double and triple refractory disease had chimeric antigen receptor (CAR) T-cell therapy-induced remission; however, those with non-refractory disease were off maintenance therapy stating they wanted relief from treatment toxicities and ‘to give my body a break’. This suggests that patients and physicians are attaching increased importance to QoL in making treatment decisions.
Within the healthcare environment, participants discussed sites of care and who was delivering care. At least 75% of participants had received care and/or treatment from a National Cancer Institute (NCI)-designated cancer center at some point since diagnosis. Participants with non-refractory disease were least likely to have interacted with an NCI-designated cancer center. Nearly all participants had received guidance from a MM specialist at some point in their care. Reasons for seeking guidance from a MM specialist included referral from a doctor, recommendation from advocacy organizations or support groups, the need for MM-specific expertise, and to ensure the best care in special circumstances (e.g., age at diagnosis, comorbidities).
In the social/cultural environment, an active MM community has arisen aided by advocacy organizations and support groups. All participants mentioned interacting with organizations or groups, which played a meaningful role in quickly dispersing current trusted information, empowering patients to advocate for themselves, and often served as an informal way to learn from MM specialists.
A combination of personal factors specific to each patient (e.g., demographics, disease aggressiveness, comorbidities) also factor into the care journey. Although not a focus of this study, there is growing momentum to improve access to care for patients. Participants acknowledged challenges to accessing expertise and care; challenges included travel logistics, out-of-pocket costs, care responsibilities for loved ones, insurance barriers, and wait times to coordinate care. Participants mentioned plans to access expertise or additional expertise if their disease progressed or if they relocated.
CONCLUSIONS
This study identified specific areas within four key environments (treatment; healthcare; social/cultural; personal factors) that are impacting the MM care journey. Of note were changes to the treatment paradigm and changing patterns of access to expert care. Participants reported that MM specialists, NCI-designated cancer centers, advocacy organizations, and support groups played an important role in pursuit of personalized care. Understanding the evolving care journey is necessary to support patients with MM in a changing landscape and to optimize treatment success.
Disclosures
Flora:Pfizer Inc: Research Funding; Seagen Inc: Research Funding. Byrd:Pfizer Inc: Research Funding; Seagen Inc: Research Funding. Platt:Pfizer Inc: Research Funding; Bristol Myers Squibb: Research Funding. Hlavacek:Pfizer Inc: Current Employment, Current equity holder in publicly-traded company. Goldman:Pfizer Inc: Current Employment, Current equity holder in publicly-traded company. Cappelleri:Pfizer Inc: Current Employment, Current equity holder in publicly-traded company. Kennedy:Adaptive Biotechnologies: Consultancy; Genentech Inc: Speakers Bureau; Bristol Myers Squibb: Speakers Bureau; Pfizer Inc: Speakers Bureau. LeBlanc:Agios: Consultancy, Honoraria, Speakers Bureau; American Cancer Society: Research Funding; Deverra Therapeutics: Research Funding; Astellas: Consultancy, Honoraria, Speakers Bureau; CareVive: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Dosentrx: Current equity holder in private company; BMS/Celgene: Consultancy, Honoraria, Research Funding, Speakers Bureau; Incyte: Honoraria, Speakers Bureau; Duke University: Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding; UpToDate: Patents & Royalties; BlueNote: Consultancy, Honoraria; BeiGene: Consultancy, Honoraria; Jazz Pharmaceuticals: Research Funding; Flatiron: Consultancy, Honoraria; Meter Health: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Genentech: Consultancy, Honoraria; Lilly: Consultancy, Honoraria; GSK: Consultancy, Honoraria, Research Funding; Agilix: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria, Research Funding, Speakers Bureau; Leukemia and Lymphoma Society: Research Funding; National Institute of Nursing Research/National Institutes of Health: Research Funding; Seattle Genetics: Research Funding; Servier: Consultancy, Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal